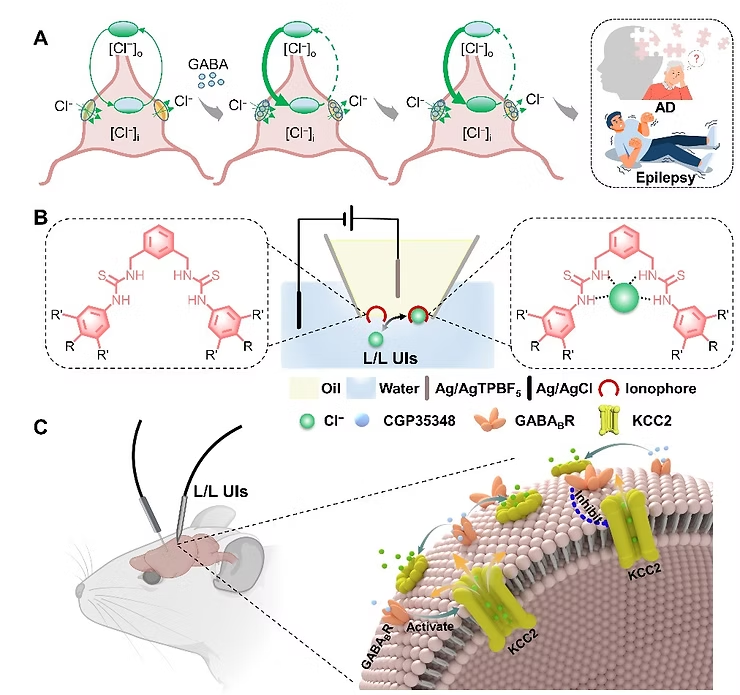
Schematic diagram of the design of L/L UIs and its dynamic tracing of CI- in the living brain of rodents (Image by SEN Liang).
Dynamic monitoring of inhibitory neural signals in brain tissue is a challenging task. Researchers have tried to track key substances involved in the neural inhibition process (e.g., Cl-) in order to tackle this problem. However, Cl- is a non-electrochemically active substance under physiological conditions and cannot easily undergo redox reactions based on electron transfer. As a result, Cl- has not been a good candidate for use in monitoring inhibitory neural signals.
Now, however, researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences have developed molecularly tailored liquid/liquid interfacial ultramicro iontronics (L/L UIs) for in vivo dynamic tracking of cerebral chloride (Cl-) regulation.
This is the first time L/L UIs have been used to achieve highly sensitive, anti-interference, reversible, real-time dynamic tracking of non-electrochemically active Cl- under physiological conditions. In this study, the L/L UIs were used for dynamic monitoring of Cl- in the brains of an Alzheimer’s mouse model and epilepsy rat model.
“Unlike conventional electronics that use electrons as signal carriers, iontronics use ions as signal carriers, which represents a novel human-machine interface,” said Prof. BAI Shuo from IPE.
The L/L-UIs developed by the researchers consist of an ultra-micropipette with an organogel-filled tip, which forms a liquid/liquid interface with brain tissue after implantation. By modifying the liquid/liquid interface with a series of bis-thiourea ionophores (IPECl-1, IPECl-2, IPECl-3) that recognize Cl-, the researchers constructed ultramicro iontronics that can monitor Cl- under physiological conditions. Under the action of these ionophores, Cl- undergoes facilitated ion transfer reaction at the interface, thereby generating detectable electrical signals.
The researchers implanted the L/L UIs into specific brain regions (such as the hippocampus, striatum, and cortex) of Alzheimer’s model mice and epilepsy model rats. They then explored the differences in Cl- concentrations between different brain regions. The L/L UIs showed high sensitivity, excellent anti-interference, and repeatability in real-time dynamic tracking of Cl- in the living brains of the rodents.
Through this dynamic tracking of live rodent brains, the L/L UIs demonstrated the regulatory role of potassium-chloride cotransporter 2 (KCC2)—which plays an important role in the neuroinhibitory process—on the concentration of Cl- in the brain.
This work is highly relevant to the field of neuroscience and has potential diagnostic and therapeutic implications for neurodegenerative diseases such as Alzheimer’s disease and epilepsy, according to a peer reviewer for Science Advances. “[It] provides new ideas for tracking non-electrochemically active ions and monitoring inhibitory neural signaling in brain tissue,” said the peer reviewer.