Researchers in Japan have made a major breakthrough in solar fuel technology, developing nanosized, porous oxyhalide photocatalysts that deliver record performance in hydrogen generation and carbon dioxide conversion. The innovation could pave the way for scalable, eco-friendly fuel production powered by sunlight.
Breakthrough in Photocatalyst Efficiency
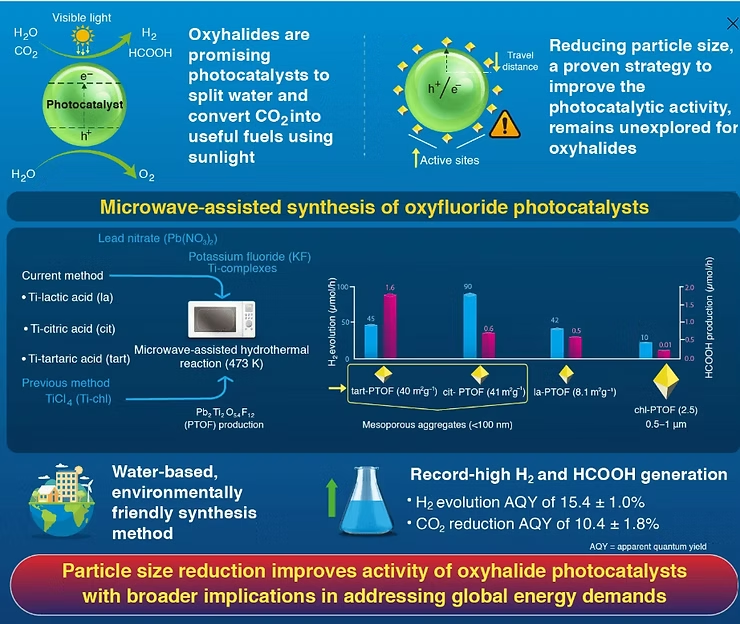
A team led by Professor Kazuhiko Maeda (Institute of Science Tokyo) and Professor Osamu Ishitani (Hiroshima University) engineered lead-based oxyhalide particles—Pb₂Ti₂O₅.₄F₁.₂ (PTOF)—with sizes under 100 nanometers and highly porous structures. Compared to conventional oxyhalide catalysts, the new materials demonstrated up to 60 times higher activity in producing hydrogen from water and converting carbon dioxide into formic acid.
Why PTOF Stands Out
Lead-based oxyhalides like PTOF are promising photocatalysts due to their:
- Narrow bandgap for strong visible-light absorption
- Resistance to oxidation, enabling long-term stability
- Adaptability for various artificial photosynthesis reactions
When exposed to sunlight, PTOF generates electrons and holes that drive reactions such as water splitting (H₂ production) and CO₂ reduction to formic acid, a liquid fuel and hydrogen carrier.
Innovative Low-Temperature Synthesis
Using a microwave-assisted hydrothermal method at just 473 K, the researchers prepared PTOF with lead nitrate, potassium fluoride, and different water-soluble titanium complexes (citric, tartaric, and lactic acid-based). This approach yielded nanosized particles with surface areas around 40 m²/g, compared to just 2.5 m²/g for conventionally made PTOF from TiCl₄, which produced much larger (0.5–1 μm) particles.
READ ALSO: Potatoes Evolved Separately from Tomatoes Around 9 Million Years Ago
READ ALSO: IKO’s New High-Thrust Linear Motor Stage Delivers Long Stroke Lengths in Constrained Spaces
Record-Breaking Performance
- Hydrogen Generation: Citric acid-derived PTOF achieved a quantum yield of ~15% at 420 nm, with reaction rates 60 times higher than conventional PTOF.
- CO₂ Reduction: Tartaric acid-derived PTOF produced formic acid with a quantum yield of ~10% in the presence of a molecular ruthenium photocatalyst—both figures being record highs for oxyhalide materials.
Interestingly, while the smaller particles had lower charge carrier mobility than larger ones, the short travel distance to the surface minimized electron-hole recombination, enhancing overall efficiency.
READ ALSO: UZ Brussel Reaches Breakthrough in Robotic Microsurgical Treatment of Lymphedema
Towards Scalable Solar Fuel Production
The environmentally friendly, low-temperature process offers a practical pathway for scaling up artificial photosynthesis. “Controlling the morphology of oxyhalides is key to unlocking their full potential as photocatalysts,” said Maeda. “Our findings could play a significant role in developing materials that address global energy challenges.”
This achievement marks a major step toward harnessing solar energy not only for electricity but also for clean, sustainable fuel production—bringing large-scale artificial photosynthesis closer to reality.