Sodium-ion batteries (SIBs) are increasingly recognized as one of the most promising candidates for large-scale energy storage systems (ESS). Their appeal lies in several advantages: sodium is abundant and inexpensive compared with lithium, the raw material costs are significantly lower, and the systems generally provide higher safety. With the demand for renewable energy storage growing rapidly, sodium-ion technology has the potential to complement or even rival lithium-ion batteries in certain applications.
One of the key factors in enhancing the performance of SIBs is the choice of cathode menergyaterial. Among the options available, layered transition metal oxides have attracted strong research interest. They offer several benefits, including relatively simple synthesis routes, high specific capacity, and good sodium-ion conductivity. Within this group, the P2-type layered oxide structure stands out. It has the potential to deliver higher energy density by activating lattice oxygen redox (LOR) reactions. However, this advantage comes at a cost: LOR often triggers irreversible phase transitions, which lead to structural distortions, slower sodium-ion diffusion, and ultimately poor cycling stability. These drawbacks have been a major bottleneck in the widespread adoption of P2-type oxides.
Innovative Aluminum Substitution Strategy
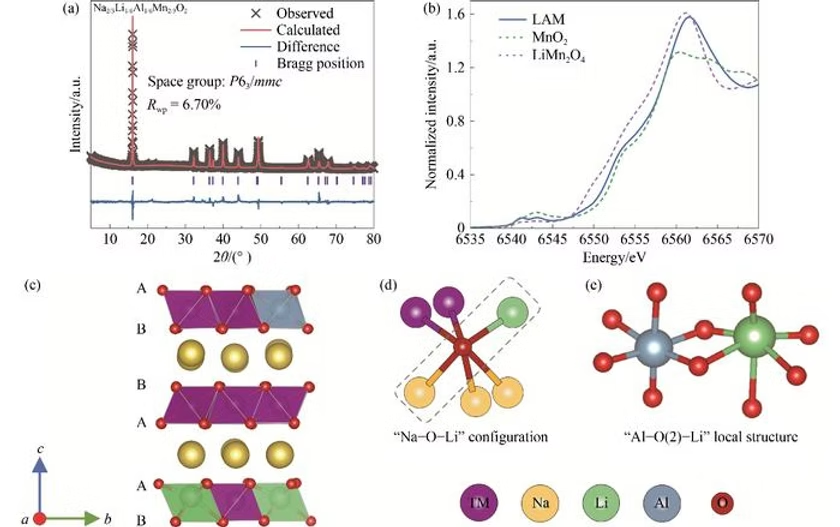
In a recent study, researchers from Shanghai Jiao Tong University, Fudan University, and Brookhaven National Laboratory presented an innovative solution to this problem. They proposed an aluminum (Al) substitution strategy to stabilize the P2-type oxide structure. By introducing flexible Al–O bonds into the compound Na2/3Li1/6Al1/6Mn2/3O2 (LAM), the team successfully suppressed the formation of unstable O-type stacking.
Instead, they promoted the generation of a more stable Z phase, which has lower energy barriers and provides smoother sodium-ion pathways. This approach also alleviated local structural stress, leading to a significant improvement in sodium-ion diffusion kinetics.
Material Synthesis and Structural Analysis
The team prepared the Al-substituted P2-type layered oxide electrode through a solid-state sintering method, a widely used technique in battery material synthesis. Advanced characterization confirmed the structural integrity of the material. X-ray diffraction (XRD) and Rietveld refinement revealed that the LAM material maintained the typical P63/mmc symmetry, with aluminum, lithium, and manganese uniformly distributed in the transition metal layers.
Further insights came from X-ray absorption spectroscopy (XAS). During the first charging process, the manganese (Mn) valence state remained unchanged, suggesting that the charge capacity originated entirely from oxygen redox. On discharge, some of the Mn(IV) ions were reduced to Mn(III), resulting in a mixed valence state of Mn(III)/Mn(IV).
Meanwhile, in situ XRD monitoring provided valuable information about the phase transition behavior. When charged beyond 4.2 volts, the material shifted from the P2 phase to the Z phase, avoiding the usual O-type stacking. The orthorhombic prism structure of the Z phase maintained the stability of sodium-ion diffusion channels, ensuring better electrochemical performance.
Enhanced Electrochemical Performance
Electrochemical testing demonstrated the effectiveness of this substitution strategy. The Al-doped electrode achieved a reversible capacity of 86 mAh/g even at a relatively high current density of 1 A/g. Moreover, it showed a capacity retention rate of 62.5% after 100 cycles, outperforming many conventional P2-type cathode materials. These results highlight how Al–O bonds play a critical role in enhancing both cycling stability and rate capability.
To further understand the mechanism, the researchers employed density functional theory (DFT) calculations combined with crystal orbital Hamilton population (COHP) analysis. The results revealed that the Al–O bonds exhibit moderate covalency and high flexibility. During sodium insertion and extraction, these bonds undergo slight contractions and distortions in the octahedral structure. This flexibility helps to relieve local structural stress, thereby reducing the sodium-ion migration energy barrier to 0.47 eV. A lower barrier directly translates into faster diffusion kinetics, which is essential for high-rate performance.
Scientific Significance and Future Potential
This study represents an important advancement in sodium-ion battery research. By demonstrating the controllable generation of the Z phase through aluminum substitution, the researchers provided new insights into how structural instabilities caused by lattice oxygen redox can be mitigated. Flexible Al–O bonds not only prevent irreversible phase transitions but also stabilize the overall local structure, ensuring smoother ion movement and improved electrochemical properties.
Looking ahead, this strategy could inspire further innovations in the design of cathode materials for sodium-ion batteries. The insights gained may also extend to other layered transition metal oxides used in different battery chemistries. With ongoing global efforts to develop sustainable, cost-effective, and scalable energy storage technologies, such breakthroughs bring sodium-ion batteries closer to becoming a viable alternative for grid storage and other large-scale applications.
READ ALSO: https://www.modernmechanics24.com/post/new-quantum-sensing-technology-reveals-subatomic-signals